Frontiers in Neurogenesis
, February 2020
Brain Structural Plasticity: From Adult Neurogenesis to Immature Neurons
Chiara La Rosa 1,2 , Roberta Parolisi 1 e Luca Bonfanti 1,2*
Brain structural plasticity is an extraordinary tool that allows the mature brain to adapt to environmental changes, to learn, to repair itself after lesions or disease, and to slow aging. A long history of neuroscience research led to fascinating discoveries of different types of plasticity, involving changes in the genetically determined structure of nervous tissue, up to the ultimate dream of neuronal replacement: a stem cell-driven “adult neurogenesis” (AN).
Yet, this road does not seem a straight one, since mutable dogmas, conflicting results and conflicting interpretations continue to warm the field. As a result, after more than 10,000 papers published on AN, we still do not know its time course, rate or features with respect to other kinds of structural plasticity in our brain.
The solution does not appear to be behind the next curve, as differences among mammals reveal a very complex landscape that cannot be easily understood from rodents models alone. By considering evolutionary aspects, some pitfalls in the interpretation of cell markers, and a novel population of undifferentiated cells that are not newly generated [immature neurons (INs)], we address some conflicting results and controversies in order to find the right road forward.
We suggest that considering plasticity in a comparative framework might help assemble the evolutionary, anatomical and functional pieces of a very complex biological process with extraordinary translational potential.
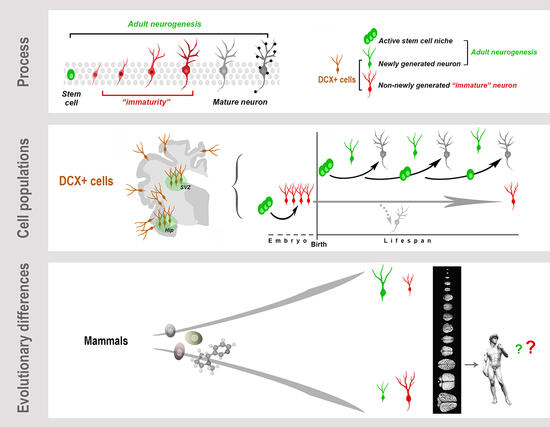
Graphical Abstract. Neurogenesis (present both in the embryonic and adult brain) is a multistep biological process spanning from the division of stem/progenitor cells to the functional integration of new neurons in neural circuits. “Immaturity” is a phase in this process, also occurring in cells that are generated before birth but retain molecular features of “youth” during adulthood. These immature neurons (INs) share markers with newly born neurons. All these cells express doublecortin (DCX), which therefore cannot be considered a unique marker for neurogenic processes. Present knowledge suggests that, despite the common cellular/molecular features shared among mammals, more complex processes, such as some forms of brain plasticity, may differ remarkably, with a general trend of reduced adult neurogenesis (AN) from rodents to large-brained species, and possible inverse