PNAS -
Proceedings of the National Academy of Sciences
24 March 2020
Cerebellar plasticity and associative memories are controlled by perineuronal nets
Carulli D 1,2,3 , Broersen R 4,5 , de Winter F 6 , Muir EM7, Mešković M 4 , de Waal M 6 , de Vries S 6 , Boele HJ 5 , Canto CB 4,5 , De Zeeuw CI 8,5 , Verhaagen J 1,9
Significance
Understanding mechanisms underlying learning and memory is crucial in view of tackling cognitive decline occurring during aging or following neurological disorders. The cerebellum offers an ideal system to achieve this goal because of the well-characterized forms of motor learning that it controls. It is so far unclear whether cerebellar memory processes depend on changes in perineuronal nets (PNNs). PNNs are assemblies of extracellular matrix molecules around neurons, which regulate neural plasticity. Here we demonstrate that during eyeblink conditioning (EBC), which is a form of cerebellar motor learning, PNNs in the mouse deep cerebellar nuclei are dynamically modulated, and PNN changes are essential for the formation and storage of EBC memories. Together, these results unveil an important mechanism controlling motor associative memories.
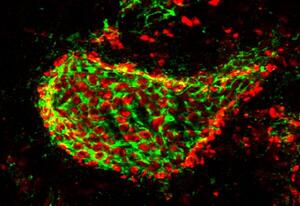
Perineuronal net (in green, stained by Wisteria floribuda agglutinin) surrounding a mouse cerebellar neuron. In the holes of the net are pre-synaptic terminals (in red, stained by antibodies against VGAT, which is expressed in axon terminals GABAergic neurons).
Abstract
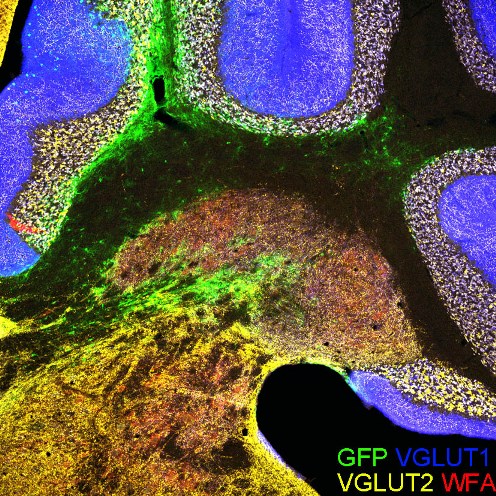
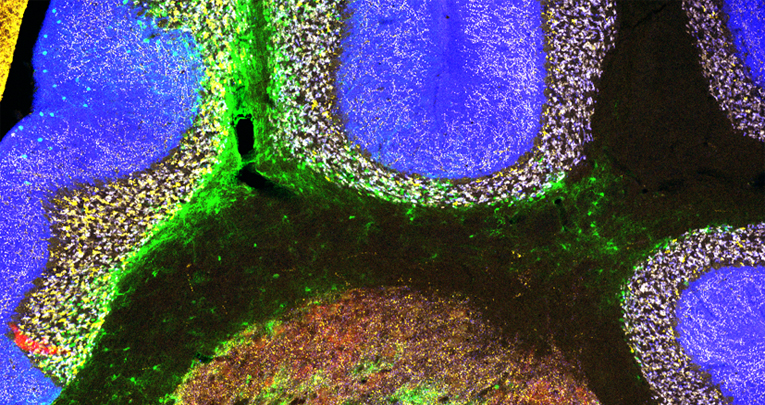
Perineuronal nets (PNNs) are assemblies of extracellular matrix molecules, which surround the cell body and dendrites of many types of neuron and regulate neural plasticity. PNNs are prominently expressed around neurons of the deep cerebellar nuclei (DCN), but their role in adult cerebellar plasticity and behavior is far from clear. Here we show that PNNs in the mouse DCN are diminished during eyeblink conditioning (EBC), a form of associative motor learning that depends on DCN plasticity. When memories are fully acquired, PNNs are restored. Enzymatic digestion of PNNs in the DCN improves EBC learning, but intact PNNs are necessary for memory retention. At the structural level, PNN removal induces significant synaptic rearrangements in vivo, resulting in increased inhibition of DCN baseline activity in awake behaving mice. Together, these results demonstrate that PNNs are critical players in the regulation of cerebellar circuitry and function.