Letizia Marvaldi brings the prestigious Rita Levi Montalcini fellowship to NICO - University of Turin
Dr. Marvaldi joined the
Department of Neurosciences Rita Levi Montalcini, University of Turin
, where she will teach
Human Anatomy
at the Chemistry and Pharmaceutical Technology course.
Here at NICO - University of Turin she joined the
Brain development and Disease Research Group
, lead by the Scientific Director prof. Alessandro Vercelli.
Dr. Letizia Marvaldi completed her master degree in Neurobiology at the University of Turin . In her master thesis, which was completed in Geuna’s lab, she was studying the peripheral nervous system’s intrinsic capacity for regeneration. After that she continued her research abroad. Initially, she moved to Innsbruck to do her PhD in Neuroscience at the international SPIN doctoral school. In the PhD project, they discovered that the reduction of the Sprouty2 gene in the sensory neurons promotes outgrowth in vitro and in vivo, using the sciatic crush lesion.
Next step lead dr. Marvaldi to the laboratory of Mike Fainzilber at the Weizmann Institute in Israel , where they discovered that importin alpha3 regulates the pain signaling pathway . This study was published in the Science journal in 2020 .
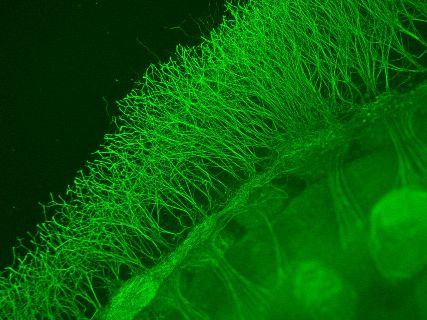
Image of an organotypic culture embryonic stage E13.5 stained with a neuronal marker in the DRG neurons (green).
Recently, she won the prestigious Rita Levi Montalcini fellowship that allowed her to return to Italy: « I am enthusiastic about the opportunity to teach my skills and abilities to young researchers. Moreover - she said - I am really happy to come back and start my research at NICO with my new fantastic colleagues» .
Dr. Marvaldi joined the Brain development and Disease Research Group at NICO - University of Turin , lead by the Scientific Director prof. Alessandro Vercelli.
Dr. Marvaldi and her team at NICO will carry out two lines of research
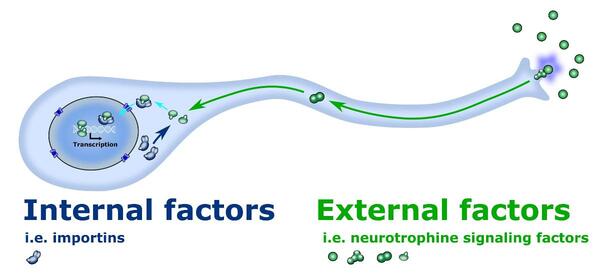
Peripheral sensory neurons must innervate the target tissue. In this process neurons receive external signals that guide them. « We already know much about those external signals but little about the interactions between the internal and external factors so that’s why I am going to investigate it! » explains dr. Marvaldi.
She aims to close this knowledge gap by culturing embryonic sensory neurons and apply neurotrophins as external factors and then knock down importin alpha3 as an internal factors which is an important internal factor. Importins are a gate-keeper to the nucleus means are determining what goes to the nucleus and changes gene expression. The research group will discover the gene expression, measure neurite outgrowth, protein localization and find the proteins or transcription factors that block neurite outgrowth.
Schematic representation of the cross-talk between intrinsic and extrinsic mechanisms in the embryonic sensory neurons
Dr. Marvaldi is also interested in how neuropathic pain is modulated by gender, aging, and social interactions . Research into these interesting interactions will unlock novel approaches to personalized pain therapy.
Regarding her research at the NICO, dr. Marvaldi will have several collaborations with Franziska Rother and Michael Bader ( Max Delbrück Center for Molecular Medicine Berlin, Germany) , Mike Fainzilber ( Weizmann Institute, Israel ), Ted Price ( Center for Advanced Pain Studies at the University of Dallas, USA ) and Michaela Kress ( Medical University Innsbruck, Austria ).
Project 1
Importins are nucleus-transporter proteins that act as master regulators of neuronal intrinsic factors. Importins are transporting signals from the periphery to the nucleus of dorsal root ganglion (DRG) neurons. We hypothesize that during development, importins determine the active transcriptional networks and thus growth and differentiation phenotypes in the sensory neurons .
Our preliminary data in developing DRG neurons demonstrates that lack of importin alpha3 changes the neurite outgrowth growth pattern in vitro as in vivo. With comprehensive RNAseq data in hand, together we will now set out to functionally characterize the underlying transcriptional networks and their functional roles of importin alpha3 in nociceptors and proprioceptors neurons.
We will determine the interaction of intrinsic factors (i.e.: importins) and extrinsic signals (i.e.: neurotrophin signaling) in patterning the phenotype of sensory neurons during development. Our efforts will lead to a comprehensive model of DRG development that will identify key drivers of neuronal differentiation and maturation. These insights can then be leveraged for regenerative or palliative medicine, as we demonstrated for chronic pain.
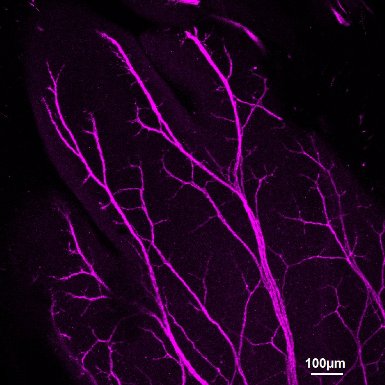
Confocal image of a dorsal hindpaw E14.5 stained with a neuronal marker (magenta).
Project 2
Neuropathic pain is a burden for our society. Circa 20 % of the population in the western world is afflicted by it which has a significant socio-economic impact. Neuropathic pain is caused by miswiring and abnormal organ targeting where excessive branching leads to more pain. We found that the loss of Importin alpha3 alleviates neuropathic pain (Marvaldi et al., 2020).
We want to exploit this importin alpha3 phenotype to improve regeneration and to reduce pain by promoting re-innervation. With a comprehensive dataset of differentially expressed genes in hand, we will thoroughly mine for the transcriptional networks and key regulators that carry the signature of axonal growth. These candidates will be functionally validated in DRG neuronal culture and in sections. Positively confirmed candidates will be further evaluated in vivo using the SNI model, to demonstrate better regeneration and less pain. Candidate genes will be knocked-down and/or rescued using an AAV9 php.S virus in wt and knock-out mice, respectively.
Finally, we will compare our genes of interest with human neuropathic pain patients’ dataset to verify if these genes exert similar functions in humans. To therapeutically exploit these findings, we will bioinformatically search for therapeutic compounds that carry the same gene expression signature as the genes that promote re- innervation. These compounds have the potential to provide the same effect as a genetic intervention but without the associated risks.