Nature Communication , 28 April 2022
Not all oligodendrocyte precursors are created equal
Enrica Boda* 1,2 ,Martina Lorenzati 1,2 , Roberta Parolisi 1,2 , Brian Harding 3 , Gianmarco Pallavicini 1,2 , Luca Bonfanti 2,4 , Amanda Moccia 5 , Stephanie Bielas 5 , Ferdinando Di Cunto 1,2 , Annalisa Buffo 1,2
The brain is a complex and fascinating organ. Part of this complexity lies in the heteroegeneity of brain cells. Since several years now it has been understood that neurons are not all the same, but have differences that make them contribute in a different and specific way to the functioning of the nervous system and that make them more or less vulnerable during aging or in the case of pathology. However, it is not yet clear whether and to what extent glial cells - oligodendrocytes, astrocytes and microglia, i.e. the non-neuronal cells of the nervous system - are heterogeneous and to what extent this can have an impact on the physiology or pathology of the central nervous system (CNS).
In a recent work published in the prestigious journal Nature Communications , NICO researchers Enrica Boda, Martina Lorenzati, Roberta Parolisi, Annalisa Buffo ( Physiopathology Group of Cerebral Stem Cells ), Gianmarco Pallavicini and Ferdinando di Cunto ( Embryonic Neurogenesis Group ), and Luca Bonfanti ( Adult Neurogenesis Group ), in collaboration with the research group of Dr. Stephanie Bielas (University of Michigan, USA) and with Dr. Brian Harding (University of Pennsylvania and Children's Hospital of Philadelphia, USA), addressed this question by focusing on the oligodendrocyte progenitors, also called OPCs.
NICO researchers involved in the research, from the left: Roberta Parolisi, Gianmarco Pallavicini, Martina Lorenzati, Annalisa Buffo, Ferdinando di Cunto, Enrica Boda e Luca Bonfanti
OPCs are the cells that give rise to oligodendrocytes, i.e. the CNS cells that produce myelin, necessary to ensure the faithful and fast conduction of signals between neurons. One of the major aspects of OPC heterogeneity is their different developmental origin: during the development of the CNS, different populations of OPCs are generated starting from different "niches" and at different times .
In spite of this different origin, in the adult brain, the OPC populations do not show apparent differences. Whether and to what extent the different developmental origin of OPCs can affect their functioning or fate in pathological conditions had never been studied, although this is a relevant question, since OPCs and oligodendrocytes are the specific targets of some of the most common pathologies of the SNC.
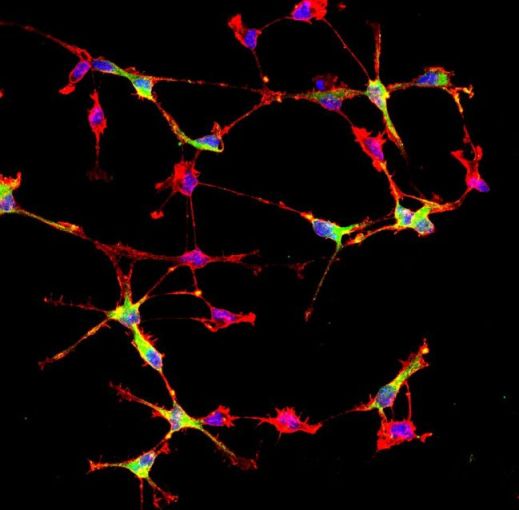
OPCs in culture. The green labeling identifies OPCs of dorsal origin, which are more vulnerable to DNA damage
In this context, the researchers found that, based on their different origins, OPCs 'inherit' a hidden diversity, latent until these cells happen to accumulate DNA damage. This hidden inheritance consists of a different ability to activate antioxidant responses and thus to survive in the event of damage.
Since DNA damage contributes to the aging of all cells and, in a primary or secondary way, to most CNS pathologies, this finding represents an important step forward for theunderstanding of the behavior of OPCs upon aging and in pathological conditions and, hopefully, for the design of new therapy approaches.
Nature Communications
, 28 April 2022
Molecular and functional heterogeneity in dorsal and ventral oligodendrocyte progenitor cells of the mouse forebrain in response to DNA damage
Enrica Boda* 1,2 ,Martina Lorenzati 1,2 , Roberta Parolisi 1,2 , Brian Harding 3 , Gianmarco Pallavicini 1,2 , Luca Bonfanti 2,4 , Amanda Moccia 5 , Stephanie Bielas 5 , Ferdinando Di Cunto 1,2 , Annalisa Buffo 1,2
1
Department of Neuroscience Rita Levi-Montalcini, University of Turin, Turin, Italy
2
Neuroscience Institute Cavalieri Ottolenghi (NICO), University of Turin, Regione Gonzole, 10 – 10043 Orbassano (Turin), Italy
3
Pathology and Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania and Children’s Hospital of Philadelphia, Philadelphia, USA
4
Department of Veterinary Sciences, University of Turin, Italy
5
Department of Human Genetics, University of Michigan, Ann Arbor, MI, USA
*Corresponding author