Neural Regeneration Research
, 25 March 2021
Searching for alternatives to brain regeneration
Chiara La Rosa 1 , Luca Bonfanti 2
Brain regeneration from an evolutionary perspective: Brain regeneration (the full restoration of tissue after loss from injury or disease) is the most sought after goal for researchers working in developmental neurobiology. It also appears to be the most challenging to achieve when considering the mammalian brain.
Whereas remarkable regenerative capacities can be present in the central nervous systems of many non-mammalian vertebrates (e.g., fish, amphibians), these kinds of processes appear to be dramatically reduced in mammals (Bonfanti, 2011). The reasons for such differences across animal classes are not completely understood, yet, some clear aspects have emerged from the study of well-established models like the teleost fish brain (Lange and Brand, 2020), which has: i) multiple, widespread stem cell niches that provide continuous, physiological cell renewal, as well as regeneration after lesioning; ii) additional neural elements that can de-differentiate after injury and re-acquire stem cell properties; iii) the ability to re-activate developmental programs in order to provide regenerative capacity.
Studies on regeneration in various tissues and organs across animal species indicate that physiological and lesion-induced regeneration requires the coexistence of some (if not all) of the above-mentioned aspects, which, in the mammalian brain, are either absent or restricted to very small neurogenic niches. The most intuitive explanation for differences in brain regeneration across animal classes, apart from causal reasons, is the need for more neuroanatomical complexity linked to increased computational capabilities that often occurs in parallel with increased brain size.
The “complexity” of large brains appears to be incompatible with substantial cell renewal/regeneration, a process that would be biologically expensive and somehow in contrast with the requirement for “stability” of the neural circuits (e.g., to retain long-term memories related to multiple previous experiences in long-living organisms). The current state of knowledge is still a mix of evidence and theories that are blurred by the frequently irregular patterns of evolution, but it does point to an important, underestimated issue: phylogenetic variations in the location, amount, rate, and type of brain plasticity in mammals.
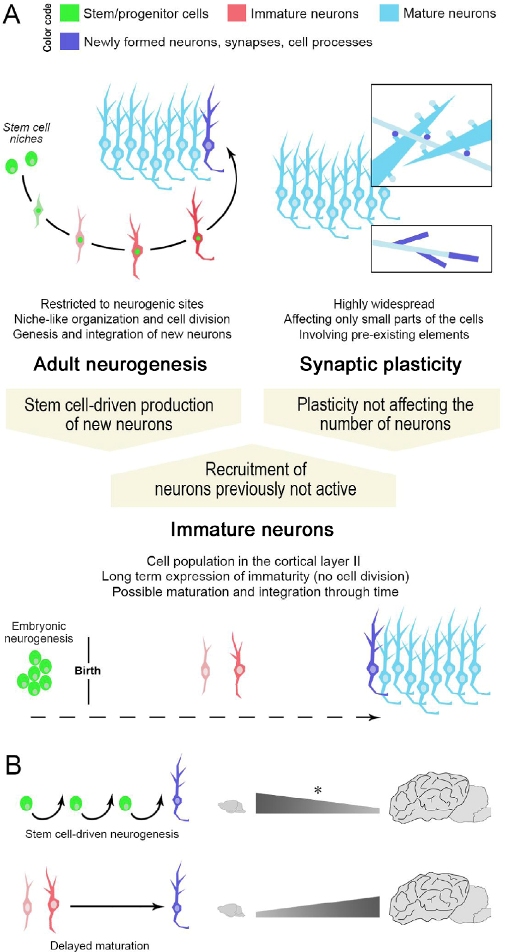
Figure 1: Different ways for achieving structural plasticity in the adult mammalian brain. Stem cell-driven genesis of new neurons (adult neurogenesis) and synaptic/axonal plasticity (A, top) represent two extremes of plastic events in the brain. Non-newly generated “immature” neurons (A, bottom), as a form of delayed neurogenesis without division, might be considered as an intermediate form of plasticity providing new elements for the pre-existing neural circuits in the absence of active stem cell niches/neural progenitors. Note that a similar outcome (the addition of a new neuron in the circuits) can be obtained through different plastic processes, not all of which involve stem/progenitor cells (high top: color code indicating different maturational states of neurons; dark blue indicates newly formed elements). (B) Specific types of plasticity such as “classic” adult neurogenesis (top) or “immature” neurons (bottom) can coexist, yet, with highly different distributions and amounts. The numbers of immature neurons can vary remarkably in mammals, with phylogenetic variation between small-brained and large-brained species (La Rosa et al., 2020b). Asterisk, the reduction in adult neurogenesis rates across mammalian species has not yet been assessed through systematic, comparable approaches.
1
Neuroscience Institute Cavalieri Ottolenghi (NICO), Orbassano, Italy
2
Neuroscience Institute Cavalieri Ottolenghi (NICO), Orbassano; Department of Veterinary Sciences, University of Turin, Grugliasco, Italy