Aging Cell, October 2016
Beta-amyloid 1-42 monomers, but not oligomers, produce PHF-like conformation of Tau protein
Manassero G 1,2,3 , Guglielmotto M 1,2 , Zamfir R 2 , Borghi R 3 , Colombo L 4 , Salmona M 4 , Perry G 5 , Odetti P 3,6 , Arancio O 7 , Tamagno E 1,2 , Tabaton M 3
The mechanistic relationship between amyloid β1-42 (Aβ1-42) and the alteration of Tau protein are debated. We investigated the effect of Aβ1-42 monomers and oligomers on Tau, using mice expressing wild-type human Tau that do not spontaneously develop Tau pathology.
After intraventricular injection of Aβ1-42, mice were sacrificed after 3 h or 4 days. The short-lasting treatment with Aβ monomers, but not oligomers, showed a conformational PHF-like change of Tau, together with hyperphosphorylation. The same treatment induced increase in concentration of GSK3 and MAP kinases. The inhibition of the kinases rescued the Tau changes. Aβ monomers increased the levels of total Tau, through the inhibition of proteasomal degradation. Aβ oligomers reproduced all the aforementioned alterations only after 4 days of treatment.
It is known that Aβ1-42 monomers foster synaptic activity.
Our results suggest that Aβ monomers physiologically favor Tau activity and dendritic sprouting, whereas their excess causes Tau pathology. Moreover, our study indicates that anti-Aβ therapies should be targeted to Aβ1-42 monomers too.
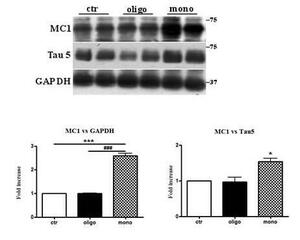
A β 1-42 monomers induce a conformational change of Tau protein.
Representative western-blot of brain extracts from control (saline) and treated (Aβ1-42) peptides by ICV for 3 hours mice (2 month-old) using a conformational Tau antibody (MC1) and a total Tau antibody (Tau5) for detection. An antibody raised against GAPDH or Tau 5 served as loading control. Densitometric quantification shows an increase of the total protein level of both MC1 and Tau5 induced by monomers. The data are mean + SEM, *p<0.05; **p<0.01 vs control by one-way ANOVA followed by Bonferroni post hoc test, n=6.